Much of my graduate research has focused around studying the mechanism of catalytic organic reactions.
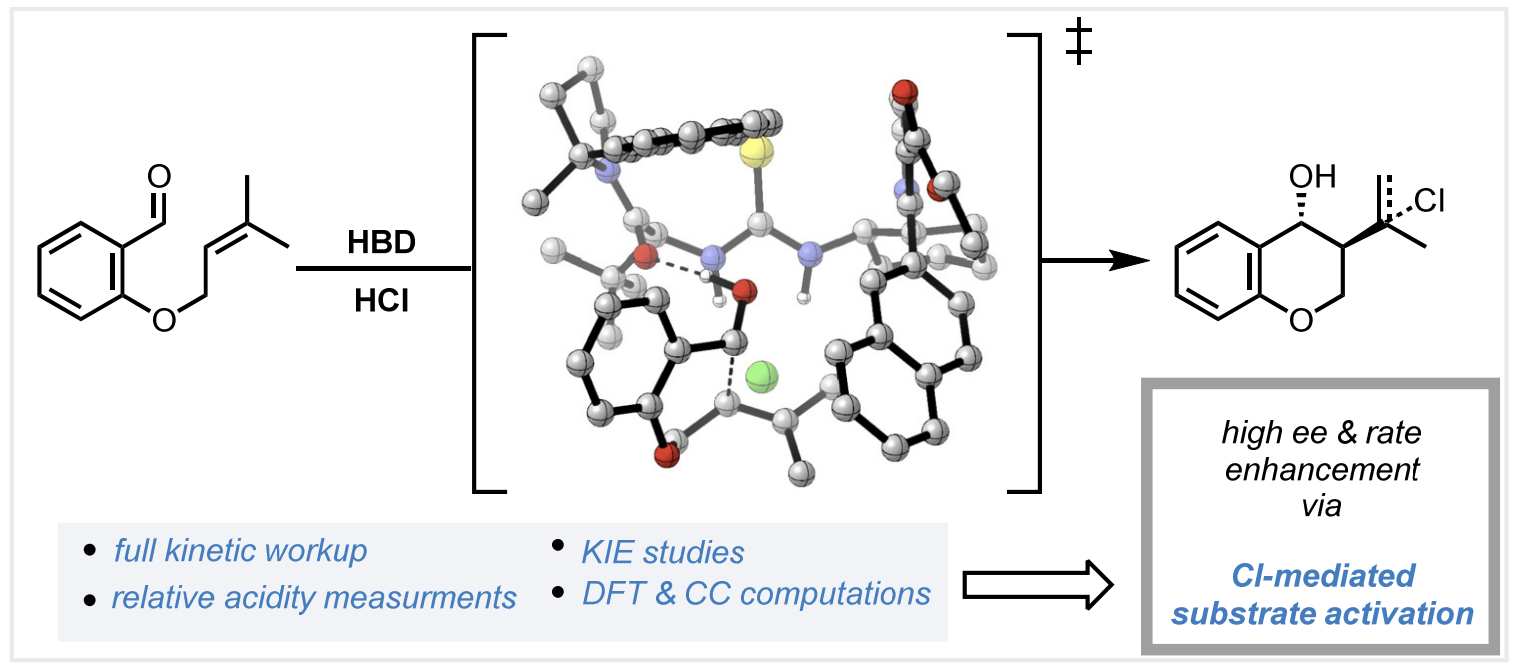
With Dennis Kutateladze, I studied the mechanism of an enantioselective acid-catalyzed Prins cyclization, motivated by the observation that hydrogen-bond donors led to a 93-fold increase in reaction rate. Surprisingly, in situ pKa measurements revealed that the catalyst actually decreases the acidity of HCl by forming a catalyst•HCl complex, opposite what one might expect. A variety of experiments and computations suggest that rate acceleration comes not from enhancement of acidity but through catalyst control of chloride positioning, which creates an electric field that stabilizes electron flow in the major transition state.
I was also part of a collaboration focused on studying the mechanism of iron-catalyzed carbonyl–olefin metathesis (COM). Long thought to proceed via a concerted [2+2] cycloaddition, our results suggest that this prediction actually arises from erroneous treatment of solvation in the computational model, and that the reaction actually goes through a carbocationic betaine intermediate. This finding brings the mechanism of Fe-catalyzed COM in line with mechanisms proposed for other COM reactions, and illustrates how poor handling of solvation can lead to deceptive results.