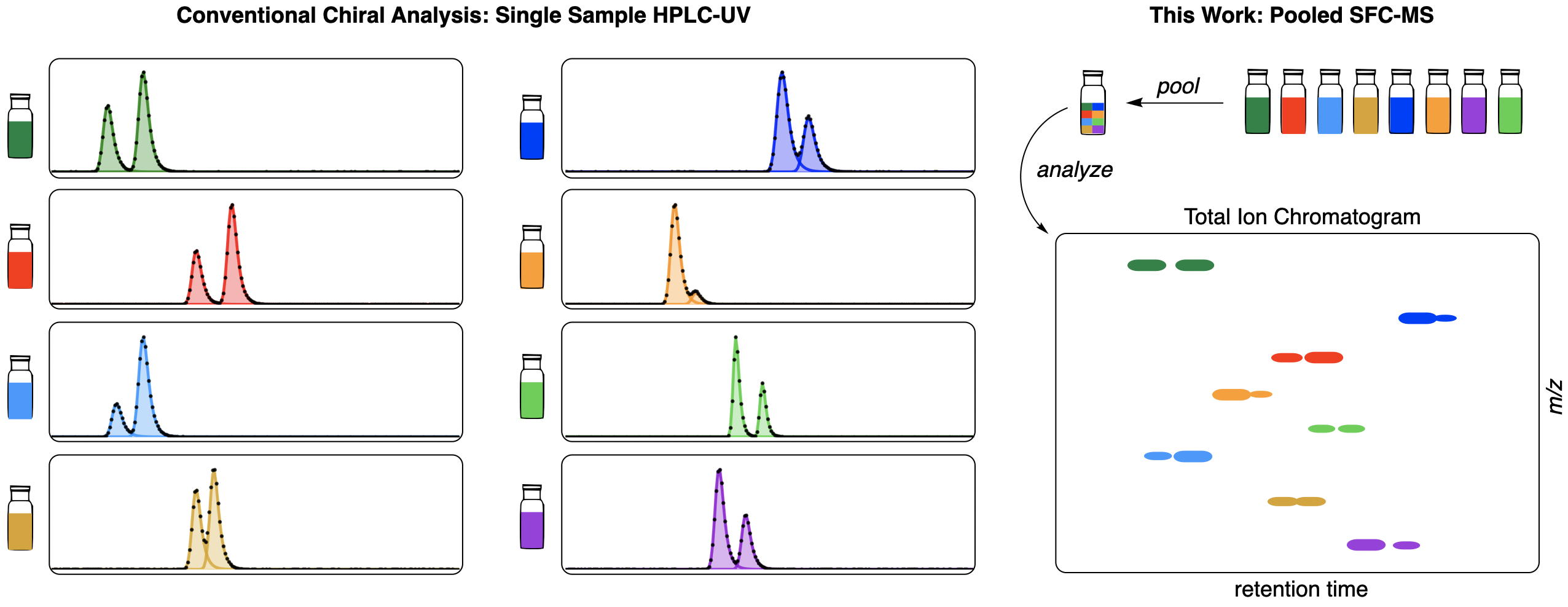
Most asymmetric reactions are optimized using a single model substrate, generating methods that often work only for a narrow range of substrates—despite the fact that generality is one of the most important factors driving adoption of new reactions. While it has been theorized for decades that screening on multiple substrates might yield more general transformations, existing enantiodetermination methods generally require pure material and substrate-specific optimization, making multi-substrate screening too costly to be practical.
We collaborated with Eugene Kwan and Spencer McMinn at Merck to try and address this analytical bottleneck. By replacing SFC-UV with SFC-MS, we were able to measure the enantioselectivity of mixtures of crude products with acceptable error, enabling rapid multi-substrate screening. We used our analytical workflow to quickly survey the generality of reported Brønsted-acid catalysts for the Pictet–Spengler reaction and found that SPINOL-derived chiral phosphoric acids gave promising results for a wide variety of substrates. Crucially, different substrates were found to show wildly divergent behavior as reaction conditions and catalysts were varied, illustrating the importance of using multiple model substrates.