Note: old versions of this post lacked a discussion of SN2. I've added an appendix which remedies this.
In “The Rate-Limiting Span,” I discussed how thinking in terms of the span from ground state to transition state, rather than in terms of elementary steps, can help prevent conceptual errors. Today, I want to illustrate why this is important in the context of a little H/D KIE puzzle.
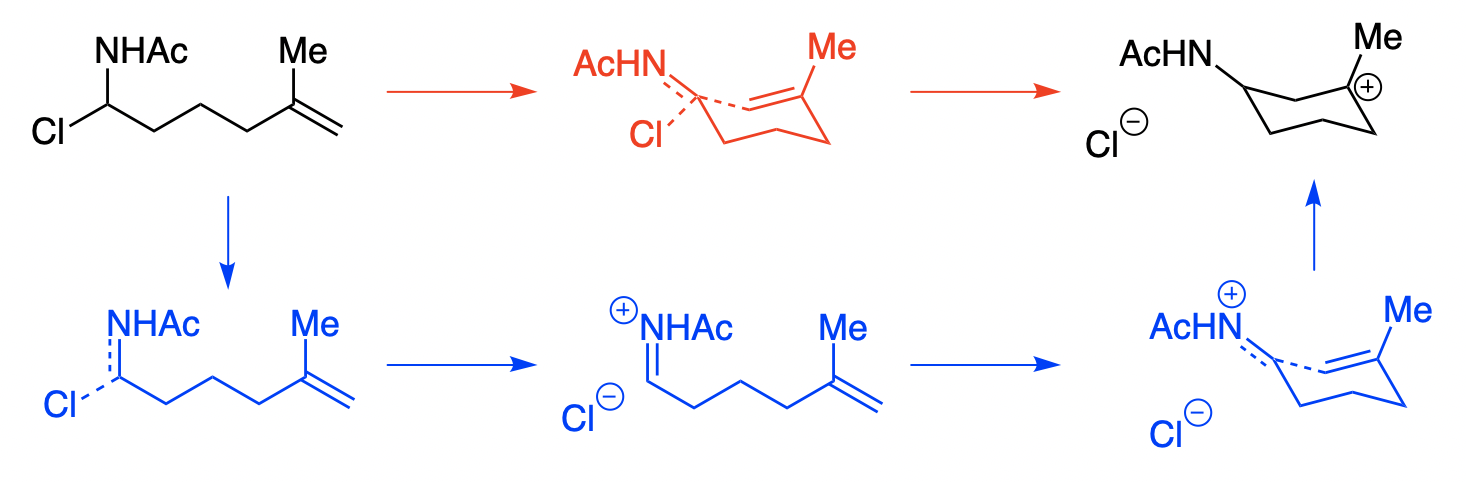
Consider the following reaction, which could conceivably proceed via an SN2 mechanism (red), an SN1 mechanism (blue), or really anywhere on the continuum:

What experiment can be used to investigate the mechanism of this reaction? One possibility is an alpha H/D KIE experiment at the iminium position. Reactions with a sp3 ground state and an sp2 transition state display secondary normal alpha H/D KIEs, while reactions with an sp2 ground state and an sp3 transition state display secondary inverse KIEs. Thus, one might think “if iminium formation is rate-limiting, the KIE will be normal, but if alkene addition is rate-limiting, the KIE will be inverse.”
Unfortunately this is not true. Instead, all mechanistic possibilities give secondary normal KIEs! I investigated this model system computationally at the wB97X-D/6-31G(d)/SMD(CH2Cl2) level of theory. Here’s a More O’Ferrall–Jencks plot of the H/D KIE at the iminium position, computed with PyQuiver (breaking bond on Y axis, forming bond on X axis):
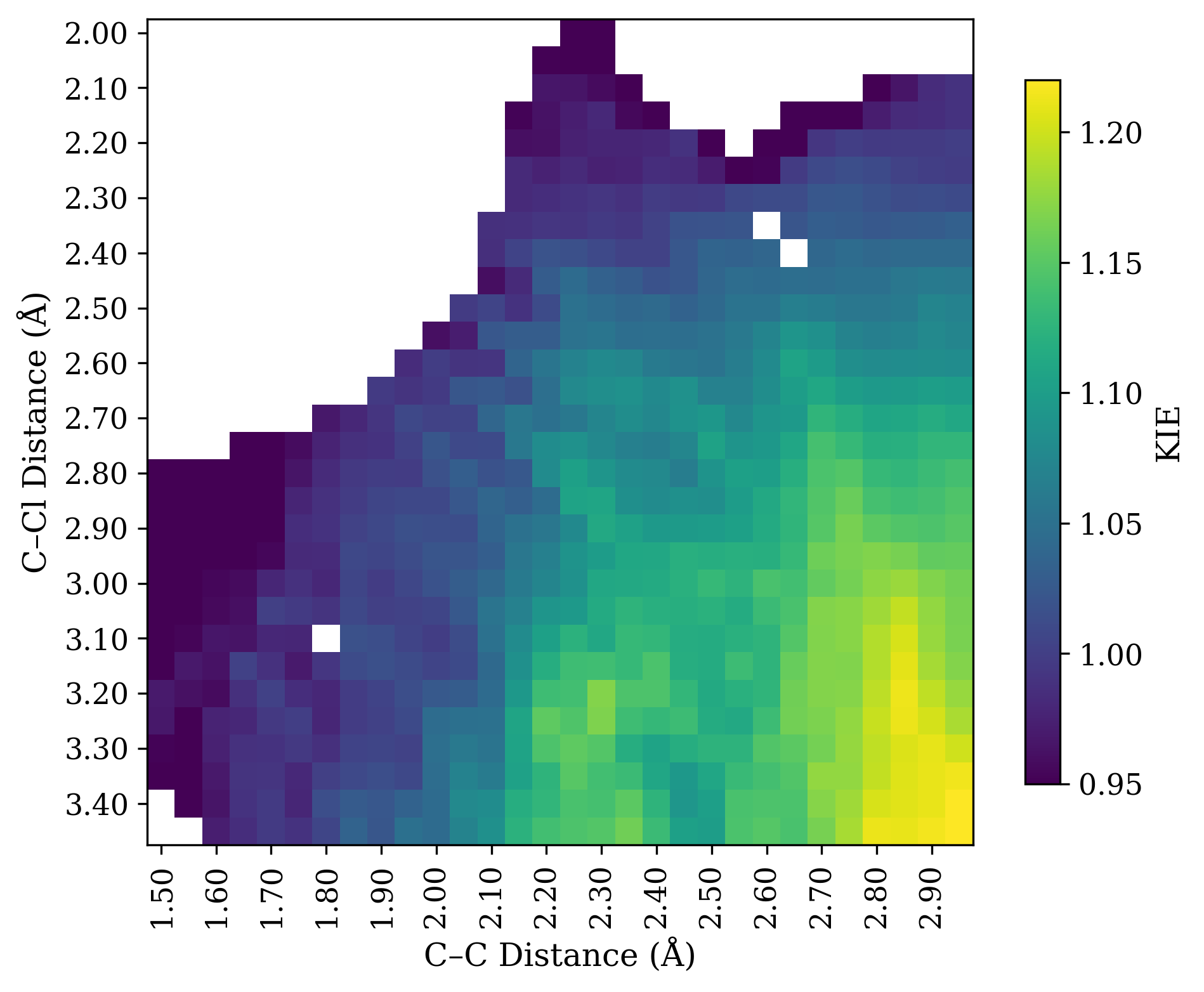
Rather than telling us which step is rate-limiting, all the KIE shows us is how much the transition state resembles an iminium ion (bottom right). Structures with long C–Cl bond distances and short C–C bond distances have substantial isotope effects (around 20%), while structures with forming or breaking bonds have smaller isotope effects.
Why is this? Both ionization and addition proceed through iminium-like structures that are substantially sp2-hybridized at carbon, irrespective of whether sp2 character is technically increasing or decreasing in the elementary step. Relative to the starting material, both transition states look like iminium ions and thus lead to large isotope effects.
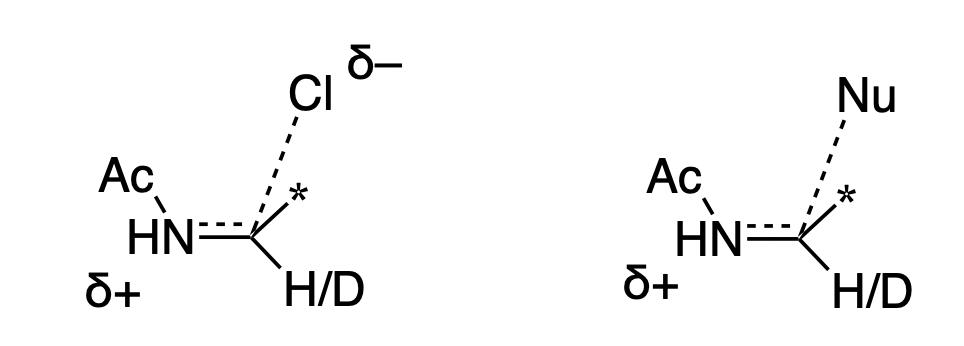
We can also conceptualize this in terms of elementary steps. Taken by itself, alkene addition does lead to an inverse kinetic isotope effect, as seen by the decreasing values as one moves left from the iminium—but an inverse isotope effect relative to the normal equilibrium isotope effect of the iminium. In this system, the equilibrium isotope effect of the iminium is larger than the kinetic isotope effect for alkene addition, and so the combination of these two effects leads to a (smaller) overall normal effect.
(This is the opposite of primary H/D KIEs, where central transition states lead to the largest isotope effects and equilibrium effects are typically small. Here, the isotope effect is mainly a function of hybridization, and so the later the TS, the greater the difference in hybridization and the larger the isotope effect.)
In summary, although this experiment seems informative, it’s actually not very useful. It tells you something about the structure of the transition state, but not which step is rate-limiting! In this case, a better experiment would be to measure 13C KIEs, or an H/D KIE on the nucleophile.
On Twitter, Mark Levin asks about the KIE for the concerted path. I originally meant to include a discussion of this, but then totally forgot to! So let’s fix that.
As shown in the graph above, extremely concerted pathways (i.e. going right down the middle of the MOJ plot) will have pretty small isotope effects. These sorts of mechanisms are common where the carbocation would be extremely unstable (methyl iodide) but much less common for stabilized carbocations like we’re discussing here. When oxocarbeniums or iminiums are involved, even “concerted” mechanisms shift towards the bottom right corner: this is the “loose”/“exploded” SN2 often seen in glycosylation. These pathways will have a modest to large H/D KIE, depending on the exact system and how “exploded” they are (see page 53 of this SI for examples)
Putting this together, then, what experimental results would be conclusive? A very small KIE would be diagnostic for a “classic” synchronous SN2 process, which I consider to be very unlikely here. But medium or large H/D KIEs are consistent with any possibility: SN1 with rate-limiting ionization, SN1 with rate-limiting nucleophilic attack, or a loose SN2. There’s an annulus of different mechanistic possibilities that all give about the same isotope effect, as shown below:
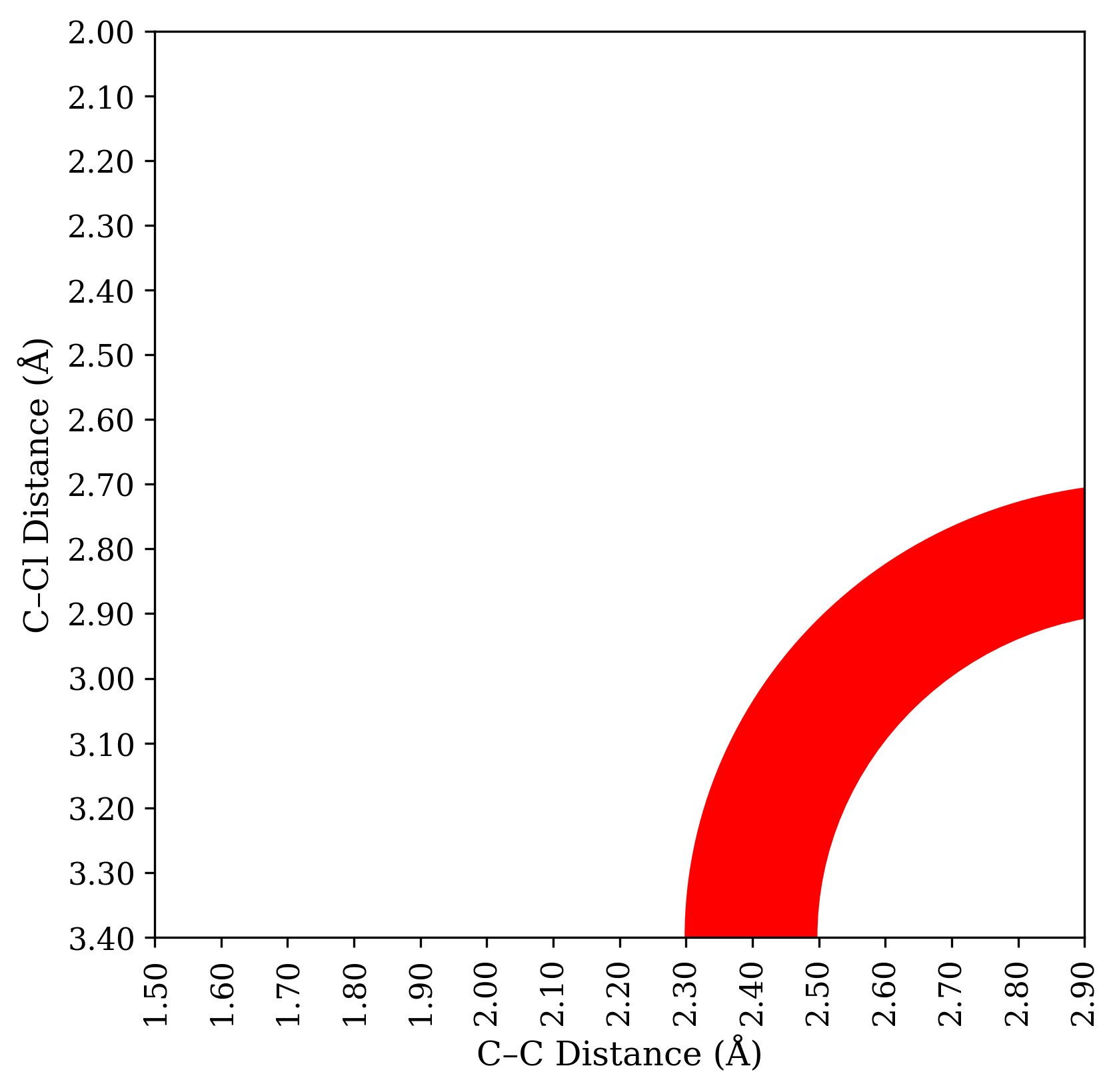
To make matters worse, H/D KIEs are pretty tough to simulate quantitatively, because of tunneling, so the annulus isn’t even that precise. That’s why I think this isn’t a very good experiment.
The raw KIE values from PyQuiver were pretty noisy, probably because there are small or multiple imaginary frequencies for some of these non-stationary points, so I used convolution to smooth things out a bit.
import numpy as np
from scipy.ndimage import convolve
#### this code block takes a 2d np.ndarray of KIE values
#### and returns a smoothed np.ndarray with the same dimensions
corner_w = 0.05
edge_w = 0.2
kernel = np.array([
[corner_w, edge_w, corner_w],
[edge_w, 1 ,edge_w],
[corner_w, edge_w, corner_w]
])
kernel = kernel / np.sum(kernel)
smooth_grid = convolve(kie_grid, kernel, mode="nearest")
I haven’t seen this technique used before, but it doesn’t seem unreasonable to me.