
Since my previous “based and red pilled” post seems to have struck a nerve, I figured I should address some common objections people are raising.
Although this is obvious, I wanted to preface all of this by saying: this is my opinion, I'm not some expert on systems of science, and many of the criticisms come from people with much more scientific and institutional expertise than me. It's very possible that I'm just totally wrong here! But what I'm saying makes sense to me, and (it seems) to a lot of other people, so I think it's at least worth having this discussion.
A few people pointed out that there are lots of instances in which carbon NMR is very important (1, 2, 3, 4, 5). I don't disagree with this at all; I've also used 13C NMR to solve problems that 1H NMR and mass spectrometry alone couldn't solve! But just because it’s crucial sometimes doesn’t mean it’s crucial all the time. Does anyone really think that you need carbon NMR to tell if Boc protection of a primary amine worked?
Most of the reactions that people do—especially people for whom synthetic chemistry is a means and not an end—are pretty straightforward, such that I think it’s fair to assume you could deduce the correct product with high confidence without 13C NMR. (Again, if carbon spectra were so crucial, it wouldn’t be the case that many people don’t get them until the very end of the project.)
This point was also made by a number of people
(1,
2,
3),
perhaps most succinctly by “Chris Farley”:

I think this is an important point—part of what we ought to do, as a scientific community, is challenge one another to test our hypotheses and scrutinize our assumptions. Nevertheless, I’m not convinced this is a particularly strong argument for carbon NMR specifically. What makes 13C{1H} spectra uniquely powerful at challenging one’s assumptions, as opposed to other data?
Keith Fritzsching points out that HSQC is much faster and gives pretty comparable information (as did other readers, privately), and simply replacing 13C with HSQC in most cases seems like it would nicely balance hypothesis testing with efficiency.
(Relatedly, Xiao Xiao recounts how reviewers will request 13C spectra even when there’s plenty of other data, including HSQC and HMBC. This is a pretty nice illustration of how powerful status quo bias can be.)
Both @VT_Chemist and Steve Fletcher made this point:
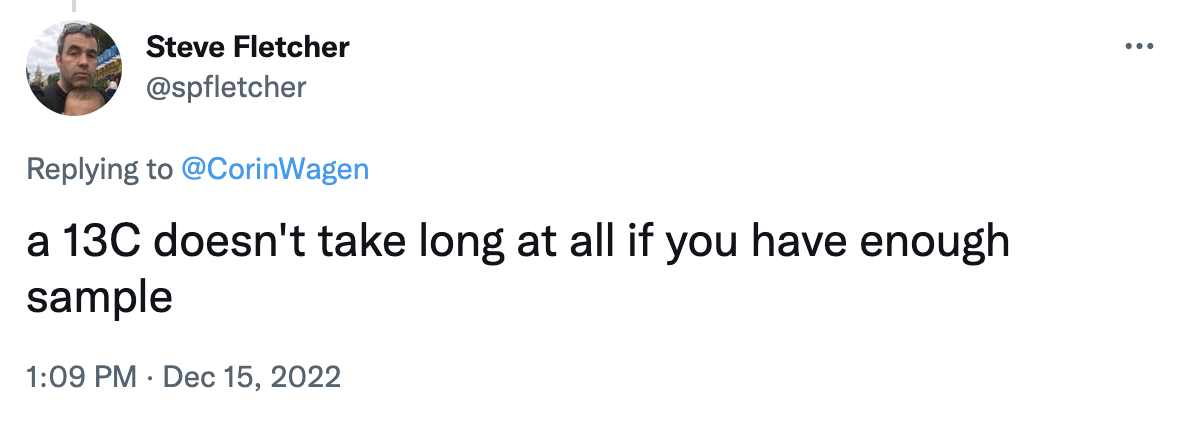
I've heard this from plenty of people before, and it's true that sometimes it's not hard at all to get a nice carbon spectrum! But sometimes it is really hard, also. Based on the other responses, it seems like lots of other people agree with this sentiment.
(Is it possible that some of this disagreement reflects whether one has access to a helium cryoprobe?)
A few people pointed out that making carbon NMR necessary on a case-by-case basis would be burdensome for editors and reviewers, since they'd have to think through each case themselves (1, 2). This is a fair point, and one I don't have a good response to.
However, it's worth noting that this is already what we do for pretty much every other claim, including many complex structural problems: give the data, draw a conclusion, and ask reviewers to evaluate the logic. Arguments about where the burden of proof should lie are tedious and usually unproductive, but I think we should have a pretty high barrier to making specific methods de jure required for publication.
I'm going to highlight Dan Singleton here, someone I respect a ton:
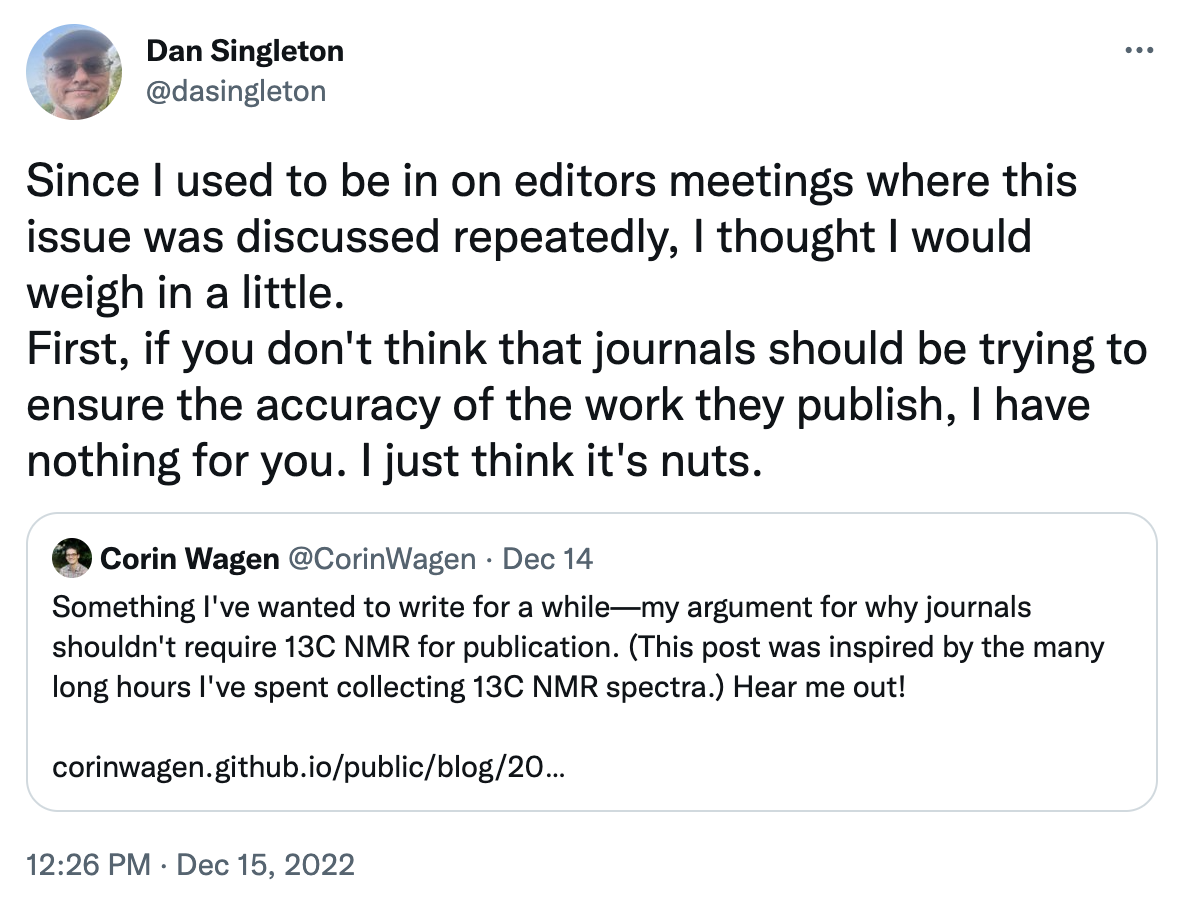
I’m not trying to suggest that journals ought not to care about accuracy at all; ceteris paribus, accuracy should be prioritized. But given that we’re all constrained by finite resources, it’s important to consider the tradeoffs we’re making with every policy decision. It’s possible that trying to increase accuracy by asking for more data could have deleterious consequences:
There’s clear extremes on both ends: requiring 1H NMR spectra for publication is probably good, but requiring a crystal structure of every compound would be ridiculous.
I think it’s easiest to think about these issues in terms of two separate questions: (1) relative to where we are today, should we push for more accuracy in the literature or less, and (2) are we achieving our chosen degree of accuracy in the most efficient manner possible?
The first question is clearly complex, and probably deserves a longer and fuller treatment that I can provide here—although I’ll note that others have espoused more radical positions than mine on peer review (h/t Michael Bogdos for the second link). I hope to write more on this subject later.
But the second question seems more straightforward. Is requiring 13C NMR for every compound a Pareto-optimal way to ensure accuracy, as opposed to HSQC or HMBC? I struggle to see how the answer can be yes.